Collaborator: J. Fuhrmann
,
K. Gärtner
Cooperation with: J. Divisek (Forschungszentrum Jülich GmbH, IWV-3)
Supported by: IWV-3 Jülich
Description:
During the second year of the project we have focused on:
- support and adaptation for use at Forschungszentrum Jülich GmbH, IWV-3,
- the extension of the CH3OH kinetics to Pt/Ru catalysts,
- a model taking into account mixed wettability by negative capillary
pressures,
- some code extensions for fuel cells using H2 rich gas mixtures,
- the energy balance equation and temperature dependence of the parameters,
- the fit of experimental data.
Short general description of a DMFC:
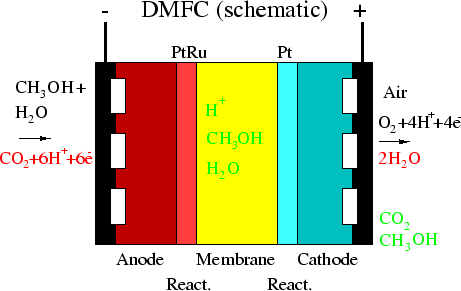 Methanol (0.5 ... 2.0 mol) dissolved in fluid water enters through
channels of
the diffusion layer (anode, left). At the PtRu catalyst grains CO2, H+, e- are
produced. The membrane transports H+, the skeleton transports e-
to contacts and at the Pt
catalyst grains the H+, e-, and O2 react to water.
This water must be removed and competes with the O2 diffusing from the
cathodic (right) channel to the reaction zone.
Methanol (0.5 ... 2.0 mol) dissolved in fluid water enters through
channels of
the diffusion layer (anode, left). At the PtRu catalyst grains CO2, H+, e- are
produced. The membrane transports H+, the skeleton transports e-
to contacts and at the Pt
catalyst grains the H+, e-, and O2 react to water.
This water must be removed and competes with the O2 diffusing from the
cathodic (right) channel to the reaction zone.
The following table summarizes the different variables present at the different
locations (gases: red, true fluids: blue, solved tracers: green,
electrochemical potentials: yellow, temperature: black, the immobile variables
describe the fraction of the occupied catalytic sites by the species produced
in different electrochemical reaction steps):
|
Layer | Mobile Species | Immob. | Composition |
| An. diff. | H2O;
CO2, CH3OH;
H2O, CO2, CH3OH;
e-;
T | 0
| graphite, teflon |
| An. react. | H2O;
CO2, CH3OH;
H2O, CO2, CH3OH;
e-, H+;
T | 8 or 10
| graphite, teflon, nafion, Pt, PtRu |
| Membrane | H2O;
CO2, CH3OH;
H+;
T | 0
| nafion |
| Cath. rea. | H2O;
CO2, CH3OH;
H2O, N2, O2;
e-, H+;
T | 8+4
| graphite, teflon, nafion, Pt |
| Cath. diff. | H2O;
CO2, CH3OH;
H2O, N2, O2;
e-;
T | 0
| graphite, teflon |
Due to methanol transport through the membrane, a parasitic reaction of
methanol takes place at the cathodic reaction layer. Depending on the width
of the reaction zone (and the concentration profile of CH3OH and O2),
the reaction may be mainly chemical or electro-chemical.
This parasitic reaction is responsible for the non-monotonic dependence
of the performance of the cell on the methanol concentration:
measurements and computations show a decreasing performance from 0.5 to
2.0 mol/l methanol concentration:
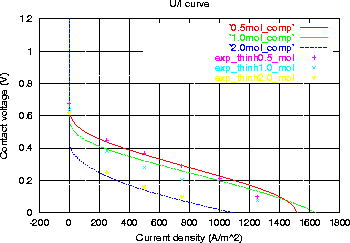
An ideal transport assumption (no methanol permeation) would yield increasing
performance with concentration.
Further experimental data are needed especially to improve the water transport
models.
To improve the overview and interaction of the different (DMFC-related)
research communities in Germany
and to present information on simulation possibilities, a workshop
focusing
on all processes around the membrane was organized at WIAS (November 23/24;
30 participants, 14 talks, 3h round table discussion, see also:
http://www.wias-berlin.de/dmfc/workshop/workshop-prog.html
http://www.wias-berlin.de/dmfc/workshop/workshop-prog.html
).
Some further details on the model equations can be found in a talk
given by J. Divisek at the Annual Congress of DECHEMA
([1]).
References:
- J. DIVISEK, R. JUNG, K. GÄRTNER, J. FUHRMANN,
Numerical simulation of Direct Methanol Fuel Cells (DMFC),
in: Proceedings of 3rd European Congress of Chemical Engineering,
Nuremberg, June 26-28 2001, Gesellschaft für Chemische Technik und
Biotechnologie e.V., Frankfurt/M., 2001.
URL:
http://www.dechema.de/veranstaltung/ecce/cd/pages/498.htm
.
- H. DOHLE, J. DIVISEK, R. JUNG,
Process engineering of the Direct Methanol Fuel Cell,
J. Power Sources, 86 (2000), pp. 469-477.
- A.A. KULIKOVSKY, J. DIVISEK, A.A. KORNYSHEV,
Two-dimensional simulation of Direct Methanol Fuel Cell:
A new (embedded) type of current collectors, J. Electrochemical
Soc., 147 (2000), pp. 953-959.
LaTeX typesetting by I. Bremer
9/9/2002
 Methanol (0.5 ... 2.0 mol) dissolved in fluid water enters through
channels of
the diffusion layer (anode, left). At the PtRu catalyst grains CO2, H+, e- are
produced. The membrane transports H+, the skeleton transports e-
to contacts and at the Pt
catalyst grains the H+, e-, and O2 react to water.
This water must be removed and competes with the O2 diffusing from the
cathodic (right) channel to the reaction zone.
Methanol (0.5 ... 2.0 mol) dissolved in fluid water enters through
channels of
the diffusion layer (anode, left). At the PtRu catalyst grains CO2, H+, e- are
produced. The membrane transports H+, the skeleton transports e-
to contacts and at the Pt
catalyst grains the H+, e-, and O2 react to water.
This water must be removed and competes with the O2 diffusing from the
cathodic (right) channel to the reaction zone.
